Could Apple expectations be any higher? The Club stock made history Tuesday, eclipsing a $4 trillion market capitalization for the first time ever, joining fellow portfolio names Nvidia and Microsoft above that level. (Nvidia on Wednesday became the first company to go above a $5 trillion valuation .) Apple shares closed just below the $4 trillion milestone Tuesday and were back and forth a session later. The remarkable comeback for Apple shares, which earlier this year were left for dead, occurred as CEO Tim Cook got on the right side of President Donald Trump ‘s desire to bring manufacturing back to the U.S., and more recently, as demand for the new iPhone models turned out to be stronger than analysts had expected. Throw in the favorable ruling in a landmark antitrust case that secures Apple’s lucrative search partnership with Alphabet’s Google, and the tech behemoth’s upcoming earnings report Thursday evening becomes one of Apple’s most pivotal in recent memory. That’s because a seemingly long-awaited iPhone upgrade cycle finally taking shape, combined with other tailwinds, have bought Apple more time to deliver on its crucial generative artificial intelligence features before its lackluster AI rollout comes back into focus. AAPL YTD mountain Apple YTD Bank of America, however, is not worried about AI. In fact, the analysts said in a Wednesday note that Apple will be an “eventual winner” in artificial intelligence. That’s why BofA thinks Apple’s earnings could double from 2024 to 2030. The analysts also increased their Apple price target to $320 per share from $270, implying an 18% upside from Tuesday’s close. Earlier this week, JPMorgan said Apple shares are “heading into the upcoming earnings print with a greater halo of positivity than any time in the past year.” The analysts cited “robust revenue and margins” that will “reinforce to investors a positive product cycle for the company.” The analysts raised their price target to $290 from $280. Despite positives, Apple naysayers continue to doubt the stock due to management’s underwhelming artificial intelligence rollout. Apple Intelligence, the iPhone maker’s generative AI suite, has faced a series of delays. Making matters worse, the company still hasn’t shared much about its roadmap despite postponing its AI-enhanced version of Siri until at least 2026. Plus, top AI talent at Apple keeps getting poached by Big Tech peers such as Club holding Meta Platforms . Still, Apple has enough going for it to shake off these AI concerns heading into the company’s earnings report. In fact, the Club sees three reasons that investors have been able to overlook the overhang. iPhone 17 demand For starters, the latest lineup of iPhone 17 models, along with the iPhone Air, has shown promising signs since their launch in September. Early iPhone 17 lead times, a gauge for consumer demand, have largely exceeded those of last year’s iPhone 16 debut. Recent data from research firms reinforce this idea, too. According to Counterpoint Research , Apple’s global smartphone shipments increased 4% year-over-year in the third quarter. The report, published on Oct. 15, also described the newest iPhone 17 series as being “well received” and having experienced “record-breaking pre-booking across regions.” Upbeat commentary from cellular providers on iPhone sales, Apple’s biggest source of revenue, has also been welcomed news. Late last month, Mike Sievert, then-outgoing CEO of T-Mobile , said the company experienced all-time high iPhone sales. “We just had the biggest iPhone week,” Sievert, who has since stepped down as chief executive, told Jim Cramer in a CNBC interview. “We’re up double digits from a year ago,” he said at the time. For the Club’s part, Jim has described the iPhone 17’s debut as “gigantic,” adding that the newest devices are “more of a bargain” than past versions. “What Apple is saying is, ‘Look, these are full price, but because you get a discount from the carriers like Mike Sievert at T-Mobile and the value of the trade-in turned out to be more than we thought.’ So, there’s been no increase in price,” Jim said in September. Baird analysts said a solid reception to the iPhone could mean better-than-expected fiscal 2025 fourth quarter results. “We continue to view AAPL as well positioned to report upside to Sep-qtr expectations and guide Dec-qtr above Street expectations later this week,” Baird said in a Tuesday note. “Our checks suggest this may be more than the average iPhone refresh cycle, as lead times for the base iPhone 17 continue to outpace last year’s levels (6+ weeks post launch).” The analysts, in turn, hiked their Apple price target to $280 from $230. Tariffs Apple is better positioned this quarter because of how management handled Trump’s tariff demands. After the White House threatened Apple with an additional 25% tariff on iPhones made outside the country in May, Cook announced in August an additional $100 billion investment into U.S. manufacturing to get on a better footing with the White House. That was a big deal for Apple, given that a lot of its manufacturing still takes place in China, which is also the company’s second-largest market. Apple has been able to mitigate a lot of these risks from tariffs, which could force the company to eat extra costs or raise device prices. The latter could dampen demand down the road. Google ruling Apple is in a better position than feared this quarter after a federal judge ruled in early September that Google could continue paying Apple billions per year to be the default search engine for the iPhone and other devices. The ruling follows a years-long battle between the Justice Department and Google over alleged violations of U.S. antitrust laws. Jim described the news as “shockingly favorable” at the time, adding it was a “big win for Apple.” The decision means that Apple can continue raking in billions for its high-margin services division with little cost associated. (The services unit garners revenues from subscriptions and licensing fees through offerings like Apple TV, iCloud, Apple Music, the App Store, and more.) Apple is estimated to have been paid $20 billion annually by Google as part of the deal. Overall, TDCowen analysts said Monday that Apple stock sentiment is “markedly positive” into quarterly earnings, in part, because these search revenue streams from Google remain intact. And, it can serve as a blueprint for AI chatbot placement. To be sure, Apple does have a high bar to hit this earnings season following a great third-quarter release in July. The company posted its biggest growth in quarterly revenue since 2021. Yet again, Apple notched an all-time high base of active devices in all of its product categories. The crucial services business hit a new record, supported by better-than-expected gross margins. Bottom line High expectations aside, Apple still seems well-positioned heading into the release. According to market data provider LSEG’s consensus estimate, Apple is expected to report earnings-per-share (EPS) growth of 8% to $1.77 in its final quarter of its 2025 fiscal year. Revenue in the three months ended in September is seen rising 7.7% year-over-year to $102.24 billion. Since the iPhone’s September launch, the Club has pounded the table on the newest line of smartphones. It’s been especially important as Apple faces an increasingly crowded smartphone market in China, too. Investors can heave a sigh of relief into the quarter, as Apple has been able to bypass two major headwinds that weighed on shares earlier this year as well: Trump’s tariff demands and the DOJ ruling. While Apple’s staggered artificial intelligence rollout is something to watch, we have said time and time again that the company doesn’t need to be the first to market to win. Why should AI be any different? What matters more is management’s track record of delivering the best, most innovative devices. Apple didn’t invent the smartphone, but the iPhone still remains the “greatest product in the world,” Jim has said. That’s why we continue to maintain our “own, don’t trade it” thesis on this high-quality stock. (Jim Cramer’s Charitable Trust is long AAPL, META, MSFT, NVDA. See here for a full list of the stocks.) As a subscriber to the CNBC Investing Club with Jim Cramer, you will receive a trade alert before Jim makes a trade. Jim waits 45 minutes after sending a trade alert before buying or selling a stock in his charitable trust’s portfolio. If Jim has talked about a stock on CNBC TV, he waits 72 hours after issuing the trade alert before executing the trade. THE ABOVE INVESTING CLUB INFORMATION IS SUBJECT TO OUR TERMS AND CONDITIONS AND PRIVACY POLICY , TOGETHER WITH OUR DISCLAIMER . NO FIDUCIARY OBLIGATION OR DUTY EXISTS, OR IS CREATED, BY VIRTUE OF YOUR RECEIPT OF ANY INFORMATION PROVIDED IN CONNECTION WITH THE INVESTING CLUB. NO SPECIFIC OUTCOME OR PROFIT IS GUARANTEED.
Blog
-
Omacetaxine and Venetoclax Show Benefits in MDS, No Improvement in AML | Targeted Oncology
A phase 1b/2 trial (NCT04874194) revealed that the combination treatment of omacetaxine and venetoclax (Venclexta) in patients with relapsed/refractory acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) with RUNX1 mutation showed…
Continue Reading
-

Enormous black hole unexpectedly found in tiny galaxy
An unexpected monster black hole was found hiding inside one of the Milky Way’s tiniest neighbors, rewriting what scientists thought they knew about how small galaxies hold themselves together.
Segue 1 is an ultra-faint dwarf galaxy located about…
Continue Reading
-
Just a moment…
Just a moment… This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
Continue Reading
-
Kraft Heinz trims annual sales, profit forecasts as demand remains stressed – Reuters
- Kraft Heinz trims annual sales, profit forecasts as demand remains stressed Reuters
- Kraft Heinz Lowers Full-Year Outlook on Weak Consumption Trends The Wall Street Journal
- Kraft Heinz’s Mixed Bag: Sales Slip While Profit Holds Up Finimize
- Kraft Heinz: Q3 Earnings Snapshot San Francisco Chronicle
- Unlocking Q3 Potential of Kraft Heinz (KHC): Exploring Wall Street Estimates for Key Metrics Yahoo Finance
Continue Reading
-
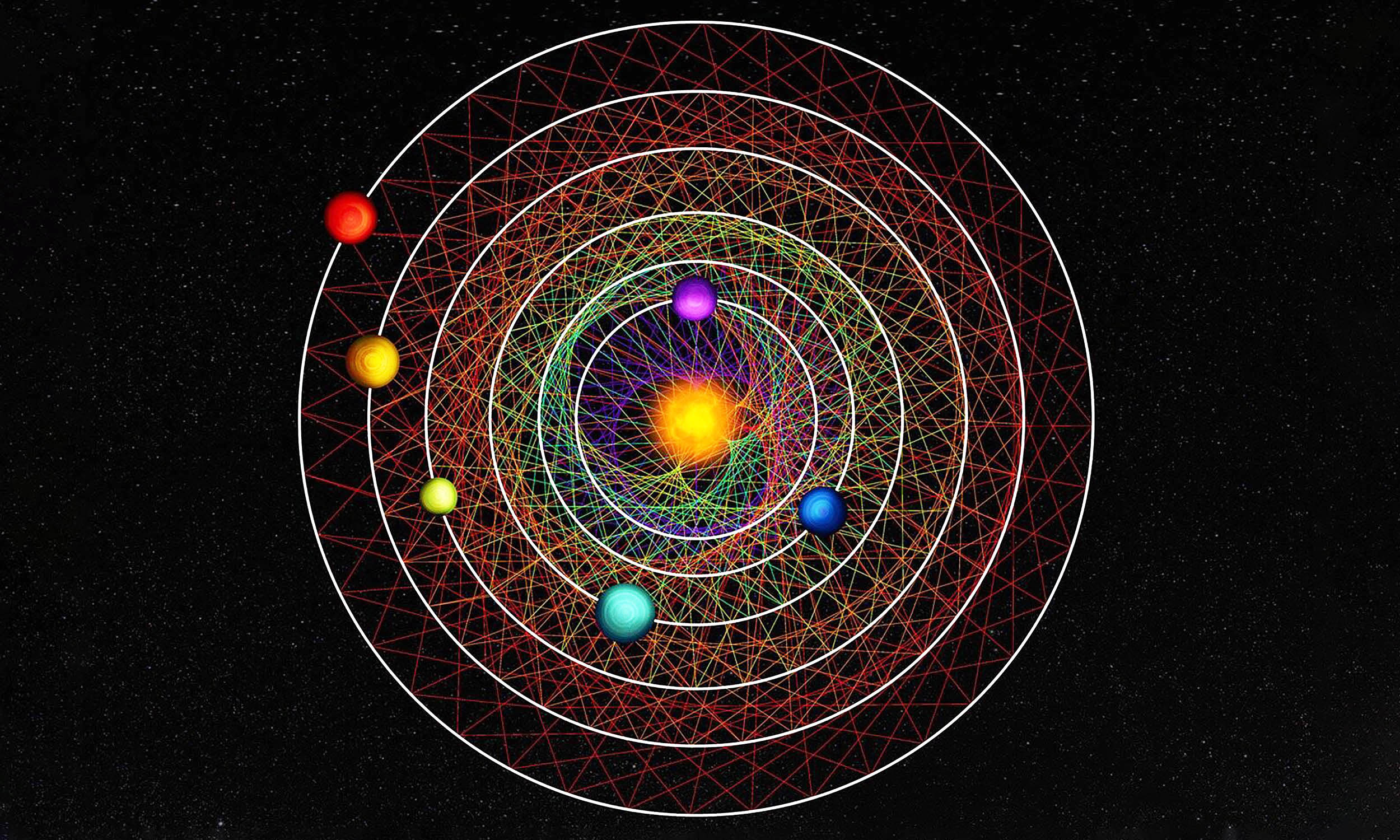
Star system found with six Neptunes in perfect mathematical orbit
Astronomers have identified a star system, named HD 110067, where six planets keep steady orbital ratios with one another. This pattern is a resonance, which means each world completes a set number of laps while its neighbor finishes a different,…
Continue Reading
-

Novartis ianalumab first drug to reduce disease activity and patient burden in Sjögren’s disease Phase III trials
- NEPTUNUS-1 and NEPTUNUS-2 achieved primary objective of reduced disease activity and provided clinically meaningful benefit1
- Data showed consistent improvements across secondary outcome measures, and a favorable safety profile1
- Novartis plans to submit to health authorities globally in early 2026
- If approved, ianalumab could become first targeted treatment for this heterogeneous, systemic autoimmune disease
Basel, October 29, 2025 – Novartis today presented new ianalumab data in Sjögren’s disease, the second most prevalent rheumatic autoimmune disease2, at a late-breaker presentation during the American College of Rheumatology Convergence congress1.
Ianalumab 300 mg monthly delivered a clinically meaningful benefit in the global NEPTUNUS-1 and NEPTUNUS-2 Phase III trials, showing both improvement in disease activity and reductions in patient burden1. Compared to placebo, ianalumab achieved a numerically greater reduction in disease activity by Week 16 with improvements sustained through Week 52 as measured by the EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI)1.
“Sjögren’s disease is a debilitating autoimmune condition affecting multiple organs causing a wide spectrum of symptoms such as dryness, fatigue, pain, and an increased risk of lymphoma – that together may create a substantial disease burden,” says Professor Xavier Mariette, Head of Department of Immuno-Rheumatology, Bicêtre Hospital, Assistance Publique – Hôpitaux de Paris, Paris-Saclay University, France. “The NEPTUNUS trials were the first Phase III studies in Sjögren’s in which a treatment significantly improved disease activity and demonstrated that ianalumab has the potential to provide a clinically meaningful benefit to patients.”
Ianalumab is a fully human monoclonal antibody with a novel dual mechanism of action that depletes B-cells and also inhibits their activation and survival via BAFF-R blockade3. B-cell dysfunction plays a significant role in Sjögren’s disease by causing an autoimmune response that leads to inflammation and tissue damage4-6.
“Today’s results reinforce our confidence that ianalumab has the potential to transform the treatment of this complex disease where no targeted medications currently exist,” said Shreeram Aradhye, M.D., President of Development and Chief Medical Officer at Novartis. “We look forward to working with health authorities globally to bring this innovation to people with Sjögren’s disease, the second most prevalent rheumatic autoimmune disease.”
NEPTUNUS study outcomes from 219 trial sites in 35 countries
The replicate NEPTUNUS trials showed statistically significant improvement in ESSDAI, the primary endpoint, at week 48 for ianalumab 300 mg monthly1. Numerical improvements were observed as early as Week 16, which were sustained throughout the study1.Patients receiving ianalumab showed consistent numerical improvements in secondary outcome measures including:
- More patients with ESSDAI low disease activity1
- Improvement in Physician Global Assessment1
- Reduction in overall disease burden as early as Week 8 continuing to Week 52 as assessed by Patient Global Assessment1
- Numerical improvement in dryness, pain and fatigue as assessed by Sjögren’s Syndrome Symptom Diary and EULAR Sjögren’s Syndrome Patient Reported Index1
- Improvement of stimulated Salivary Flow (sSF) rate and oral dryness vs placebo in patients with sSF>0.4 mL/min at baseline, in a post-hoc analysis1
Ianalumab 300 mg monthly numerically improved physician- and patient-reported outcomes1. Nominal significance was observed in NEPTUNUS-1 and the pooled data set for PhGA and PaGA, as well as the number of patients achieving low disease activity based on ESSDAI in the pooled data set1. The pooled and individual patient-reported secondary outcomes did not reach statistical significance1.
The trial results showed favorable safety with an overall incidence of adverse events and serious adverse events comparable to placebo in both studies1.
About NEPTUNUS-1 and NEPTUNUS-2
The Phase III clinical trials, NEPTUNUS-1 and NEPTUNUS-2, are global, multicenter, pivotal studies evaluating the efficacy and safety of ianalumab in patients with Sjögren’s disease7,8. These trials were designed to provide comprehensive data on the potential of ianalumab as a targeted treatment for Sjögren’s disease, in patients with active extraglandular disease3,7,8.NEPTUNUS-1 is a randomized, double-blind, 2-arm multicenter Phase III trial (N=275) designed to evaluate the clinical efficacy, safety, and tolerability of ianalumab 300 mg subcutaneous (s.c.) monthly compared with placebo for 52 weeks7. NEPTUNUS-2 is a randomized, double-blind, 3-arm multicenter Phase III trial (N=504) to evaluate the clinical efficacy, safety, and tolerability of ianalumab 300 mg s.c. monthly or every 3 months compared with placebo for up to 52 weeks8.
Patients currently enrolled in the trials have been given the opportunity to continue follow-up in these studies or enter a long-term extension trial that will continue to assess the long-term efficacy and safety of ianalumab9.
Ianalumab (VAY736) is also being investigated for its potential to treat other B-cell driven autoimmune diseases including immune thrombocytopenia (ITP), systemic lupus erythematosus (SLE), lupus nephritis (LN), warm autoimmune hemolytic anemia (wAIHA) and systemic sclerosis (SSc)3,10-14. Ianalumab originates from an early collaboration with MorphoSys AG, a company which Novartis acquired in 202415.
About Sjögren’s disease (previously called Sjögren’s syndrome)
Sjögren’s disease is a complex, systemic autoimmune disease that causes inflammation and tissue damage, impacting the entire body16. It primarily affects exocrine glands, leading to excessive dryness, with over 90% of patients experiencing dry eyes and dry mouth16,17. The disease is heterogenous and inflicts a wide range of symptoms, with patients most commonly experiencing dryness, fatigue and widespread pain, though 30-40% of patients will also show extraglandular organ involvement18,19. Extraglandular manifestation can be very diverse and can affect skin, musculoskeletal system, kidneys, lungs and other organs19. The risk of lymphoma is increased in patients with Sjögren’s18. Sjögren’s affects approximately 0.25% of the population with an estimated 50% undiagnosed 20-21. Sjögren’s is nine times more common in women than men16.Disclaimer
This press release contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements can generally be identified by words such as “potential,” “can,” “will,” “plan,” “may,” “could,” “would,” “expect,” “anticipate,” “look forward,” “believe,” “committed,” “investigational,” “pipeline,” “launch,” or similar terms, or by express or implied discussions regarding potential marketing approvals, new indications or labeling for the investigational or approved products described in this press release, or regarding potential future revenues from such products. You should not place undue reliance on these statements. Such forward-looking statements are based on our current beliefs and expectations regarding future events, and are subject to significant known and unknown risks and uncertainties. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those set forth in the forward-looking statements. There can be no guarantee that the investigational or approved products described in this press release will be submitted or approved for sale or for any additional indications or labeling in any market, or at any particular time. Nor can there be any guarantee that such products will be commercially successful in the future. In particular, our expectations regarding such products could be affected by, among other things, the uncertainties inherent in research and development, including clinical trial results and additional analysis of existing clinical data; regulatory actions or delays or government regulation generally; global trends toward health care cost containment, including government, payor and general public pricing and reimbursement pressures and requirements for increased pricing transparency; our ability to obtain or maintain proprietary intellectual property protection; the particular prescribing preferences of physicians and patients; general political, economic and business conditions, including the effects of and efforts to mitigate pandemic diseases; safety, quality, data integrity or manufacturing issues; potential or actual data security and data privacy breaches, or disruptions of our information technology systems, and other risks and factors referred to in Novartis AG’s current Form 20-F on file with the US Securities and Exchange Commission. Novartis is providing the information in this press release as of this date and does not undertake any obligation to update any forward-looking statements contained in this press release as a result of new information, future events or otherwise.About Novartis
Novartis is an innovative medicines company. Every day, we work to reimagine medicine to improve and extend people’s lives so that patients, healthcare professionals and societies are empowered in the face of serious disease. Our medicines reach nearly 300 million people worldwide.Reimagine medicine with us: Visit us at https://www.novartis.com and connect with us on LinkedIn, Facebook, X/Twitter and Instagram.
References
- Thomas GB, Xavier M, Stephanie F et al. Ianalumab demonstrates significant reduction in disease activity in patients with Sjögren’s Disease: Efficacy and safety results from two global Phase 3, randomized, placebo-controlled double-blind studies (NEPTUNUS-1 and NEPTUNUS-2). Presented at the American College of Rheumatology (ACR) Congress; October 24-29, 2025; Chicago, Illinois
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee on Selected Immune Disorders and Disability. Sjögren’s Disease/Syndrome. [Last accessed: October 2025] https://www.ncbi.nlm.nih.gov/books/NBK584486/
- Dorner T et al, Safety and Efficacy of ianalumab in patients with Sjögren’s disease: 52-week results from a randomized, placebo-controlled, Phase 2b dose-ranging study, Arthritis and Rheumatology 2025, 77(5):560-570
- Lee AY, Qi Z, Jackson KJ, et al. Self-reactive B cells are increased in all major stages of peripheral development in Sjögren’s disease. Immunology & Cell Biology 2025; 103: 401-410.
- Both T, Dalm VA, van Hagen PM, et al. Reviewing primary Sjögren’s syndrome: beyond the dryness – From pathophysiology to diagnosis and treatment. Int J Med Sci 2017; 14: 191-200.
- Cornec D, Devauchelle-Pensec V, Tobón GJ, et al. B cells in Sjögren’s syndrome: from pathophysiology to diagnosis and treatment. J Autoimmun 2012; 39: 161-167.
- ClinicalTrials.gov NCT05350072 [Last accessed: October 2025]
- ClinicalTrials.gov NCT0539214 [Last accessed: October 2025]
- ClinicalTrials.gov NCT05985915 [Last accessed: October 2025]
- ClinicalTrials.gov NCT05653219 [Last accessed: October 2025]
- ClinicalTrials.gov NCT05639114 [Last accessed: October 2025]
- ClinicalTrials.gov NCT05126277 [Last accessed: October 2025]
- ClinicalTrials.gov NCT05648968 [Last accessed: October 2025]
- ClinicalTrials.gov NTC06470048 [Last accessed: October 2025]
- Novartis to strengthen oncology pipeline with agreement to acquire MorphoSys [AG Press release]. [Press release]. Available at: Novartis to strengthen oncology pipeline with agreement to acquire MorphoSys AG for EUR 68 per share or an aggregate of EUR 2.7bn in cash | Novartis [Last accessed: October 2025]
- Negrini S et al, Sjögren’s syndrome: a systemic autoimmune disease, Clin Exp Med. 2022; 22(1): 9–25
- Maleki Fischbach-M, et al, Manifestations and management of Sjögren’s disease, Arthritis Res Ther, 2024; 26(1):43
- Mariette, Primary Sjögren’s symptoms, New England Journal of Medicine, 2018, 378;10
- Kerry Gairy et al, Burden of illness among subgroups of px with primary SjD and systemic involvement, Rheumatology 2021, Volume 60, Issue 4, April 2021, Pages 1871–1881
- Conrad N, et al, Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population-based cohort study of 22 millions individuals in the UK, Lancet. 2023;401(10391):1878-1890;
- Narváez J et al, Prevalence of Sjögren’s syndrome in the general adult population in Spain: estimating the proportion of undiagnosed cases, Sci Rep. 2020;10(1):10627
# # #
Continue Reading
-

Snapchat and Xbox Launch First AR Lens to Scan the Moon
Snapchatters and gaming fans alike, get ready for an out-of-this-world experience! To celebrate the release of The Outer Worlds 2 1 — the sequel to the first-person sci-fi RPG from Obsidian Entertainment — Xbox and Snapchat partnered to build…
Continue Reading
-
Fitbit’s Gemini-Powered Health Coach Begins Public Preview for Premium Users – eWeek
- Fitbit’s Gemini-Powered Health Coach Begins Public Preview for Premium Users eWeek
- Fitbit’s personal health coach in public preview is here blog.google
- Fitbit’s Shiny New App is Missing a Lot of Features Droid Life
- You won’t be able to use…
Continue Reading
-

Mika Hakkinen on his incredible recovery after horror crash at 1995 Australian GP
Thirty years ago, a 120mph puncture sent Mika Hakkinen’s car spinning over a high kerb and into a wall of tyres at the Adelaide Street Circuit. His McLaren did not have many of the safety devices modern F1 cars have.
Hakkinen suffered serious…
Continue Reading
